Aim of Experiment: Computational justification of the Job's method of continuous variation for the determination of the formula of complexes.
Theory: The Job's method of determination of complex-composition is based on the concept that when equimolar solutions of the metal-ion and the ligand are mixed in gradually varying volume-ratios, the conc. of the complex is highest when the volume-ratios equals complex-composition stoichiometry (ratio).
For the reaction , mM + nL ------------ Mm Ln
where M and L are the metal and ligand respectively; let the two equimolar solutions each has molarity co so that the volume fractions are fM = VM/(VM + VL) and fL = 1 – fM , and the (post-mixing) molarities of M and L are co fM and co fL . If cc is the equilibrium molarity of the complex, then clearly the equilibrium molarities of M and L are respectively (co fM – m cc) and (co fL – n cc) and so the (complex formation ) equilibrium relation is:
cc / (co fM – m cc) m (co fL – n cc) n = K (where K is the stability constant of the complex).
From this equilibrium relation, the cc value may be found in a iterative way.
So to justify Job's method in a computational way, fM is allowed to vary from 0.01 to 0.99 for a user-specified value-set of m, n, co and K and the resulting CC value is noted.
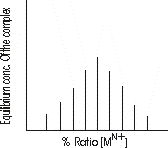
As the percentage ratio of metal-ion conc. gradually increases, the equilibrium conc. of the complex is first increases and then decreases forming a maximum, just when the ratio of the volume percentage of the metal-ion and ligand solution matches the stoichiometry of the complex as shown in the figure below:

Procedure: Using the JobJusti programme available as a part of the Computers in Chemistry Experiments Set from www.oocities.org/riturajkalita/compu_chemi.htm, the equilibrium concentration of the complex are investigated as a function of the volume percentage of the metal ion solution, for two different metal-ligand stoichiometry (1:1 and 1:4), and for two different common initial molarities (0.1 M and 0.05 M). Within the JobJusti program, the metal-ligand stoichiometry of the complex MmLn (i.e., the values of m and n), the common initial molarity, and the stability constant (equilibrium constant) of the complex to be entered, and then on pressing the 'Continue' button the in-program computation proceeds to yield the theoretical values of the equilibrium molarity of the complex as a function of the gradually varying volume percentage of the metal-ion solution (in increments of 1% from 1% to 99% ), as output to be stored in the file 'JobJusti.txt '.
Results: The results for the two different metal-ligand stoichiometry with two different initial common molarity are attached here with as photocopies of print-outs.
The maximum for equilibrium concentration of complexes occur at volume percentages as tabulated below:
| Metal co-efficient | Ligand co-efficient | Common initial molarity | Volume % for the maximal | Equilibrium molarity of complex |
|
1
|
1
|
0.05 | 50 % | 0.004196011 |
| 0.1 | 50 % | 0.019098301 | ||
| 1 | 4 | 0.05 | 20 % | 0.00000051869 |
| 0.1 | 20 % | 0.000016311727 |
Conclusion: The theoretical volume percentage ratio of metal-ion and of ligand, for maximum equilibrium molarity of the complex, exactly matches the metal-ligand stoichiometry, as is expected from the theory of Job's method. So the Job's method has been justified.