Aim of Experiment: To determine the composition of Cu (II ) and Fe (III ) in a mixed solution of the two by spectrophotometric titration using EDTA.
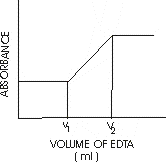
Theory: The stability constant of ferric-EDTA complex is sufficiently large as compared to that of cupric-EDTA complex. So in a mixture of Fe (III ) and Cu (II ), the formation of the latter complex starts only when Fe (III) is completely removed as ferric-EDTA complex. Moreover, the copper complex absorbs strongly at 745nm whereas at this wavelength the ferric complex does not absorb at all. The equivalence point of Fe (III) is indicated by the commencement of the formation of copper complex, when absorbance measured at 745 nm begins to increase. The equivalence point of Cu (II) is indicated by the constancy of absorbance (after arriving at a maximum). Thus a plot of absorbance vs. volume of EDTA added is obtained, to determine Fe (III ) and Cu (II) concentrations, as is shown below:
Here, V1 and V2 – V1 are the volumes of EDTA corresponding to equivalence point of Fe (III ) and Cu (II) respectively i.e.,

V1 = Fe (III)
V2 – V1 = Cu (II)
Requirements:
1) Spectrophotometer
2) Titrating flask
3) Burette
4) Standard EDTA solution (0.1 M )
5) Standard copper nitrate solution (0.02 M )
6) Standard ferric ammonium sulphate solution (0.02 M ) in 1 M H2SO4
7) Buffer solution of 1 M chloroacetic acid
8) Sodium hydroxide with pH = 2.0
Procedure: 5 ml of Cu (II) solution is mixed with 5 ml of Fe (III) solution and then 15 ml of buffer solution is added. After this the volume this mixture is diluted to 100 ml with water. Now the absorbance is adjusted to zero at the wavelength of 745 nm in the spectrophotometer and then 0.3 ml of EDTA solution is added to the mixture and the absorbance is recorded with continual stirring. The addition of EDTA is done until the recording is constant after the maximum absorbance (constancy is observed by recording constant reading after four additions).
Observation:
| Vol. of EDTA
(x, in ml) |
Observed
Absorbance (A) |
Volume-Correction
Factor F= (100 + x )/100 |
Corrected Absorbance = F.A |
Calculation: The volume of mixture = V ml
The strength of EDTA = 0.1 M
From the graph, we have
V1 ml of EDTA = Amount of Fe ( III ) in the mixture
i.e. ...........................................................................
So strength of Fe (III) in V ml mixture = 0.1V1/V
Hence amount of Fe (III) in 1000 ml =.....................
Again , (V2 – V1) ml of EDTA = Amount of Cu ( II ) in the mixture
i.e. ...........................................................................
So strength of Cu (II) in V ml mixture = 0.1(V2 – V1) / V
Hence amount of Cu (II) in 1000 ml =.....................