
By Mike Johnston
Copyright 2002
An electrical charge passing through a metallic conductor has several components, these being; (a) the current itself, which is composed of the amperage (number of electrons moving past a point per second), the voltage (the force with which the current flows) and (b) the magnetic (or electromagnetic) field which surrounds such a conductor.

Using the "Left Hand Rule" one can, from the direction of the current flow within the conductor, determine the orientation of the magnetic field around the conductor. The current direction can be determined by knowing that such current is thought to flow from the negative terminal or "pole"(in the case of a battery) where there is a surplus of free electrons, to the positive pole, where there is a lack of free electrons.
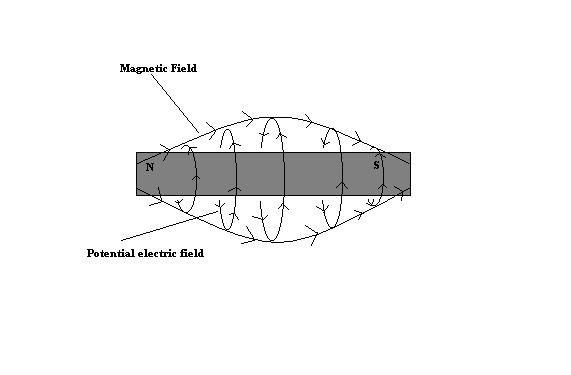
Conversely, in the field around a permanent magnet, it is the magnetic field which assumes a polar orientation , with the magnetic field (current) flowing from the north pole to the south pole and in that case the (potential) electric component could be thought of as rotating around the magnetic field. Again using the "left hand rule" (in a different way) to make this determination. So if we were to look at the two side by side we could see an obvious similarity between the to. Different sides of the same coin, if you will.

As I progressed into this research and saw how easily that the various components of the electrolysis cell could be manipulated in order to lower the internal resistance of the cell and by doing so greatly modify/improve its performance, a new idea began to form. I realized that the possibility might exist to design a cell in which the voltage from the outside power source is actually either maintained "as is" across the cell or even increased! This ability would obviously enable the production of hydrogen to proceed at a much greater level of efficiency.
I had already confirmed the fact that a small potential difference can be created in a "standard" electrolytic cell (two electrodes of the same metal) simply by changing the size ratio between the electrodes and therefore the conductivity between them and/or by causing the solution within the cell to rotate by an outside force (stirring). So from there I kind of fished around for other ways that I could modify the cell in order to favor the creation of a potential difference between the two electrodes without requiring that any additional work energy be put into the system and without having to sacrifice either of the electrodes to oxidation or reduction.
Permanent magnets came to mind as a possibility primarily
because they are indeed permanent. For all intents and purposes they never
wear out and don't need to be periodically "recharged". But could the field(s)
of permanent magnets be utilized in any kind of meaningful way in achieving
my goals? To find out I devised a series of experiments.